|
|
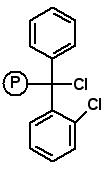
AAPPTec 2-Chlorotritylchloride Resin
|
|
|
2-Chlorotrityl chloride resin is an acid labile resin for peptide synthesis using Fmoc amino acids. The steric bulk and mild acid conditions for cleavage make 2-chlorotrityl resins useful in many applications. The steric bulk of the 2-chlorotrityl resin prevents diketopiperazide formation during the attachment of the first two Fmoc amino acids, a serious problem in the synthesis of Fmoc-Pro C-terminal and Fmoc-Pro-Xxx C-terminal peptides. Additionally, Fmoc-amino acids can be attached to 2-chlorotrityl chloride resin with essentially no racemization. Amino acid 2-chlorotrityl resins can be alternatives to Fmoc amino acid Wang resins where racemization of the first Fmoc amino acid is common as with Cys and His. Since peptide products can be cleaved from the resin with very mild acid treatment, 2-chlorotrityl chloride resin can be used to prepare protected peptide fragments for fragment condensation synthesis of large peptides and proteins.Â
2-Chlorotrityl chloride resin is moisture sensitive. It must be stored in a tightly sealed container or dessicator. If 2-chlorotrityl chloride resin is stored at low temperatures, the container must be allowed to warm to room temperature before opening. Depending on the size of the container and the amount of 2 chlorotrityl chloride resin, this could take up to two hours. For long-term storage, the 2-chlorotrityl chloride resin container should be flushed with nitrogen or argon before sealing, then the 2-chlorotrityl chloride resin should be stored at -20°C.Â
2-Chlorotrityl chloride resin and a large selection of amino acid substituted 2-chlorotrityl resins are available from AAPPTec. For a complete list of AAPPTec amino acid 2-chlorotrityl resins, click here.Â
|
|
Loading 2-Chlorotrityl Chloride Resin1
| 1. |
Use 1.0 equivalent of the first amino acid. If a lower substitution resin is required, reduce the amount of amino acid. Dissolve the Fmoc amino acid in DCM (approximately 10 mL per gram of resin). If the Fmoc amino acid does not dissolve in completely, add a small amount of DMF. (Fmoc-Asn and Fmoc-Gln are not soluble in DCM. Dissolve these amino acids in DMF then add DCM until the volume of the solution is approximately 10 mL per gram. Add a small amount of DMF if needed to obtain a clear solution.) |
| 2. |
Add the amino acid solution to the 2-chlorotrityl chloride resin. Add 1.0 equivalent (respective to the amino acid) of DIPEA. Agitate the mixture with a shaker for 5 minutes, then add 1.5 equivalents (respective to the amino acid) of DIPEA. Agitate the mixture vigorously for 30 to 60 minutes. |
| 3. |
To endcap any remaining reactive 2-chlorotrityl groups, add HPLC grade methanol, 0.8 mL per gram of resin, and mix for 15 minutes. Filter the resin and wash it three times with DCM, using approximately 10 mL per gram of resin. Wash the resin twice with DMF, twice with DCM, and three times with methanol. Dry the resin in vacuo. The substitution of the resin can be estimated from the weight gain. For an accurate measurement of the resin substitution, the amount of Fmoc released from a weighed sample of resin can be measured spectrophotometrically. |
Removing the Fmoc Group
| 1. |
To remove the Fmoc group from the first amino acid, suspend the resin in DCM/DMF/piperidine (1:1:2 by volume). Mix the suspension with a shaker for 30 minutes. Filter the resin and wash it twice with DMF. |
| 2. |
Test the resin for complete removal of the Fmoc group. Remove a small sample of resin and wash it twice with DCM to remove the DMF. Transfer the washed sample to a small test tube. Add approximately 100 microliters of 1:8 acetic acid/DCM (v/v). Let the sample stand for 30 minutes at room temperature. Evaporate the DCM and add one milliliter of 50% acetonitrile/water (v/v). Analyze the resulting solution by HPLC. There should not be any peak corresponding to the Fmoc-amino acid. |
| 3. |
If there is a significant Fmoc-amino acid peak, resuspend the resin in 1:1:2 DCM/DMF/piperidine and wait 30 minutes. Retest the resin. If necessary, repeat this process until Fmoc-amino acid is not detected. |
Â
2-Chlorotrityl Resin Cleavage1
Â
Acetic Acid Cleavage (Produces protected peptides)
|
| 1. |
Prepare a 1:1:8 by volume mixture of acetic acid/TFE/DCM (approximately 20 mL per gram of resin). Suspend the peptide 2-chlorotrityl resin in half of this mixture. Allow the mixture to stand at room temperature for 30 minutes. Filter the resin and wash it with the remaining mixture. |
| 2. |
Combine the filtrates and add 15 times the volume hexane. Remove the solvent with a rotary evaporator. If the acetic acid is not completely removed, add hexane as needed and continue evaporating the solvent. The acetic acid will be removed as an azeotrope with hexane. |
TFA Cleavage (Produces deprotected peptides)2
|
| 1. |
Remove the N-terminal Fmoc group using the procedure above. Wash the peptide 2-chlorotrityl resin three times with DCM to remove the DMF. |
| 2. |
Suspend the resin in 95% (v/v)TFA/DCM containing scavengers as required by the amino acid composition of the peptide. Allow the mixture to stand 30 minutes at room temperature. |
| 3. |
Filter the resin and wash it with a small amount of TFA. Combine the filtrates and add 8 to 10 fold cold ether to precipitate the peptide. If necessary store the mixture at 4°C overnight to complete the precipitation. |
| 4. |
Filter the peptide using a fine sintered glass funnel and wash the peptide with cold ether. |
Footnotes
|
| 1. |
Based on Barlos, K.; et al. Tetrahedron Lett. 1989, 30, 3943-3946. |
| Â |
| Â |
| AAPPTec Amino Acid 2-Chlorotrityl Resins |
| Catalog Number |
Resin |
TOP |
Go to 2-Chlorotrityl Chloride Resin protocols |
| RTA101 |
H-Ala-2-Chlorotrityl resin |
| RTR105 |
H-Arg(Pbf)-2-Chlorotrityl resin |
| RTD105 |
H-Asp(OtBu)-2-Chlorotrityl resin |
| RTN101 |
H-Asn-2-Chlorotrityl resin |
| RTN105 |
H-Asn(Trt)-2-Chlorotrityl resin |
| RTC105 |
H-Cys(Trt)-2-Chlorotrityl resin |
| RTC106 |
H-Cys(Acm)-2-Chlorotrityl resin |
| RTE101 |
H-Glu-2-Chlorotrityl resin |
| RTE105 |
H-Glu(OtBu)-2-Chlorotrityl resin |
| RTQ101 |
H-Gln-2-Chlorotrityl resin |
| RTQ105 |
H-Gln(Trt)-2-Chlorotrityl resin |
| RTG101 |
H-Gly-2-Chlorotrityl resin |
| RTH105 |
H-His(Trt)-2-Chlorotrityl resin |
| RTI101 |
H-Ile-2-Chlorotrityl resin |
| RTL101 |
H-Leu-2-Chlorotrityl resin |
| RTK105 |
H-Lys(Boc)-2-Chlorotrityl resin |
| RTM101 |
H-Met-2-Chlorotrityl resin |
| RTF101 |
H-Phe-2-Chlorotrityl resin |
| RTP101 |
H-Pro-2-Chlorotrityl resin |
| RTS105 |
H-Ser(tBu)-2-Chlorotrityl resin |
| RTS106 |
H-Ser(Trt)-2-Chlorotrityl resin |
| RTT105 |
H-Thr(tBu)-2-Chlorotrityl resin |
| RTT106 |
H-Thr(Trt)-2-Chlorotrityl resin |
| RTW105 |
H-Trp(Boc)-2-Chlorotrityl resin |
| RTY105 |
H-Tyr(tBu)-2-Chlorotrityl resin |
| RTY106 |
H-Tyr(Trt)-2-Chlorotrityl resin |
| RTV101 |
H-Val-2-Chlorotrityl resin |
|
|
|
|
| |